
Comparing Qsp and Ksp to Determine Whether a Precipitate Will Form 001
Precipitation: when Qsp > Ksp and your reaction moves backward (ions become solid) Saturation and Comparing Q vs. K sp sp Ksp represents the ion product of a saturated solution in terms of molar solubility (x). You can think of it as a measurement of the maximum saturation capacity of a solution.

Solubility, Precipitation, Ksp, Qsp, Common Ion effect YouTube
If Qsp Ksp there'stoomuchproduct thereactionwill shift towardsthereactants and precipitatewill form If Qsp Kspthere's not enoughproduct thereaction will shifttowards the products andprecipitate willnot form if Qsp Ksp thesolutionwill be saturatedbut not enough ##### to form a. precipitate ##### Ex KHCultyou s forms

How to Determine if Precipitate will Form or Not Examples, Practice
Compare, interpret and make predictions with Qsp and Ksp.Instagram: Lean.ThinkWebsite: LeanThink.org

PPT Solubility Equilibrium PowerPoint Presentation, free download
🎯 Want to ace chemistry? Access the best chemistry resource at http://www.conquerchemistry.com/masterclass📗 Need help with chemistry? Download 12 Secrets t.

Ksp, Qsp, Solubility Constant, Common Ion Effect Part 2 Grade 12
It is meaningless to compare the solubilities of two salts having different formulas on the basis of their Ks values. Example 17.2.2 17.2. 2. The solubility of CaF 2 (molar mass 78.1) at 18°C is reported to be 1.6 mg per 100 mL of water. Calculate the value of Ks under these conditions.
How To Calculate Qsp How can ksp be calculated? Download Free ePub
The solubility product constant, Ksp K s p , is the equilibrium constant for a solid substance dissolving in an aqueous solution. It represents the level at which a solute dissolves in solution. The more soluble a substance is, the higher the Ksp K s p value it has. Consider the general dissolution reaction below (in aqueous solutions):

Qsp Ksp Studyhelp
The solubility product constant ( Ksp K s p) describes the equilibrium between a solid and its constituent ions in a solution. The value of the constant identifies the degree to which the compound can dissociate in water. The higher the Ksp K s p, the more soluble the compound is. Ksq K s q is defined in terms of activity rather than.

Qsp Ksp Studyhelp
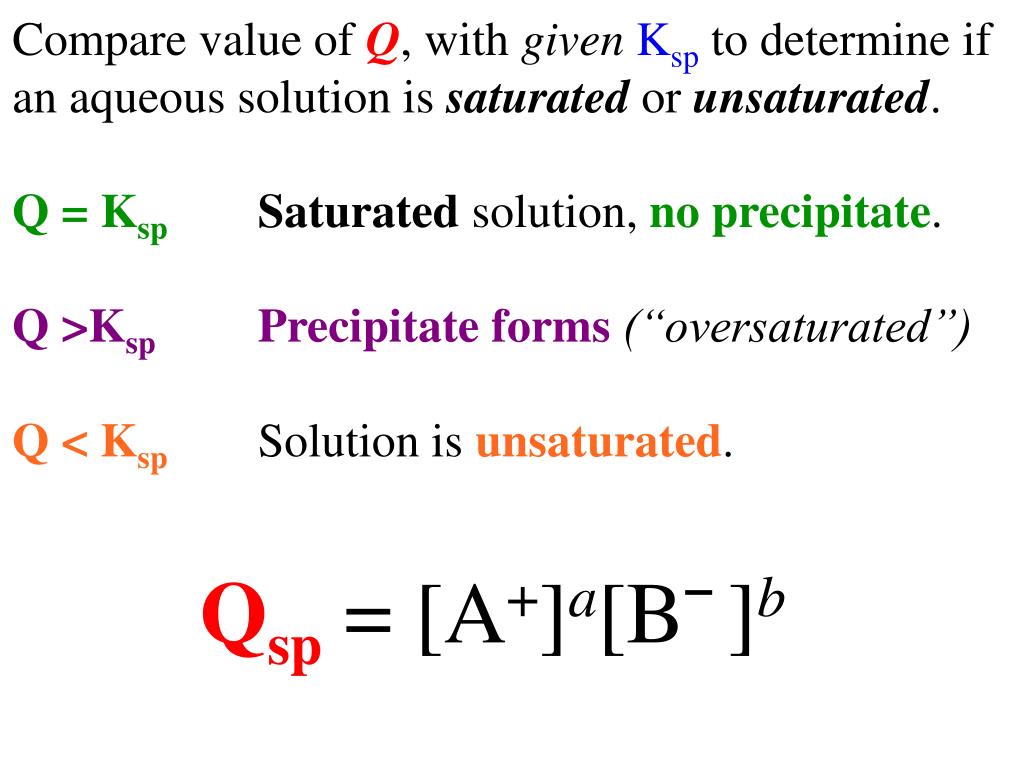
Qsp is the solubility of the ions at any concentration while Ksp is the solubility of a product of the concentration of ions at EQUILIBIRUM. Qsp= ksp---> solution is saturated at equilibrium and no precipitation will form. Qsp< Ksp---> solution is unsaturated and no precipitate will form. Qsp>Ksp--> solution is supersaturated and so precipitate.

Ksp video 3 Using Qsp to predict precipitation for AP Chemistry YouTube
If. Q = Ksp, Q = K s p, then equilibrium has been reached, and no MACROSCOPIC change will occur. If \ [\text {Q Ksp},\] then precipitation will occur. Note: Remember that the solubility product is always dependent upon the solubility equation. The solubility equation depends upon the concentration of the dissociated ions and their numbers. The.
PPT Solubility Equilibria PowerPoint Presentation, free download ID
What is the difference between Qsp and Ksp? Chemistry Chemical Equilibrium Ksp 1 Answer anor277 Feb 25, 2017 Well, Ksp is an actual equilibrium constant, that is experimentally measured. Explanation: And Q, the so-called ion product, is a TRANSIENT, non-equilibrium value.

Qsp Ksp Studyhelp
K sp is the equilibrium constant for an ionic solid dissociating into ions in water. The molar solubility is the molar concentration of the solid that dissolves/dissociates in water. It is also equal to 'x' in an ICE table. What is the molar solubility of AgCl? (K sp = 1.8×10-10). What is the molar solubility of Ag 2 S? (K sp = 6.0×10-51). The molar solubility of BiI 3 is 1.32×10-5 M.

Kelarutan Massa Endapan (Ksp dan Qsp) part 3 YouTube
The main difference between Ksp and Qsp is that Ksp is a constant value that represents the equilibrium condition for the dissolution of a salt, while Qsp is a variable that represents the current ion concentrations in a solution at any given moment.

Compare Qsp and Ksp YouTube
Qsp stands for Solubility Product Quotient and is used to describe the current state of an aqueous solution. If Qsp is less than Ksp, then more solid can be dissolved. But if Qsp is larger.
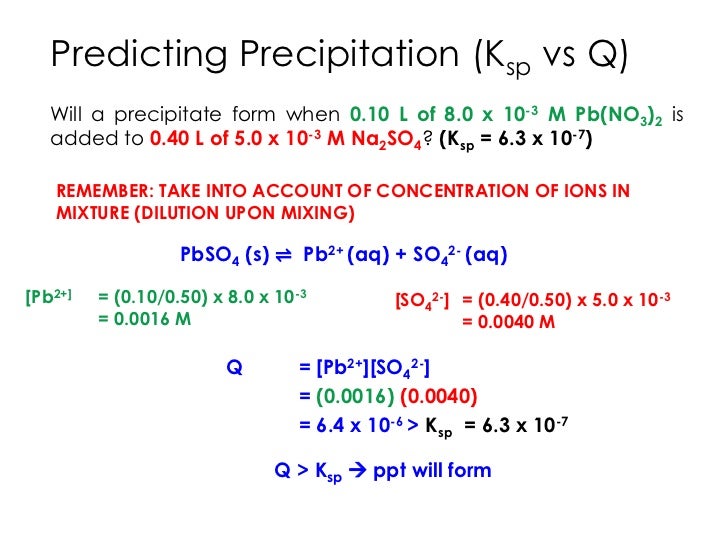
+Will+a+precipitate+form+when+L+of+0.jpg)
Qsp Ksp Studyhelp
Ksp and Qsp: Solubility Equilibria JFR Science 14.3K subscribers Subscribe Subscribed Share 16K views 6 years ago Mr. Key explains how the solubility product constant (Ksp) and the solubility.
PPT Ionic Equilibria III The Solubility Product Principle PowerPoint
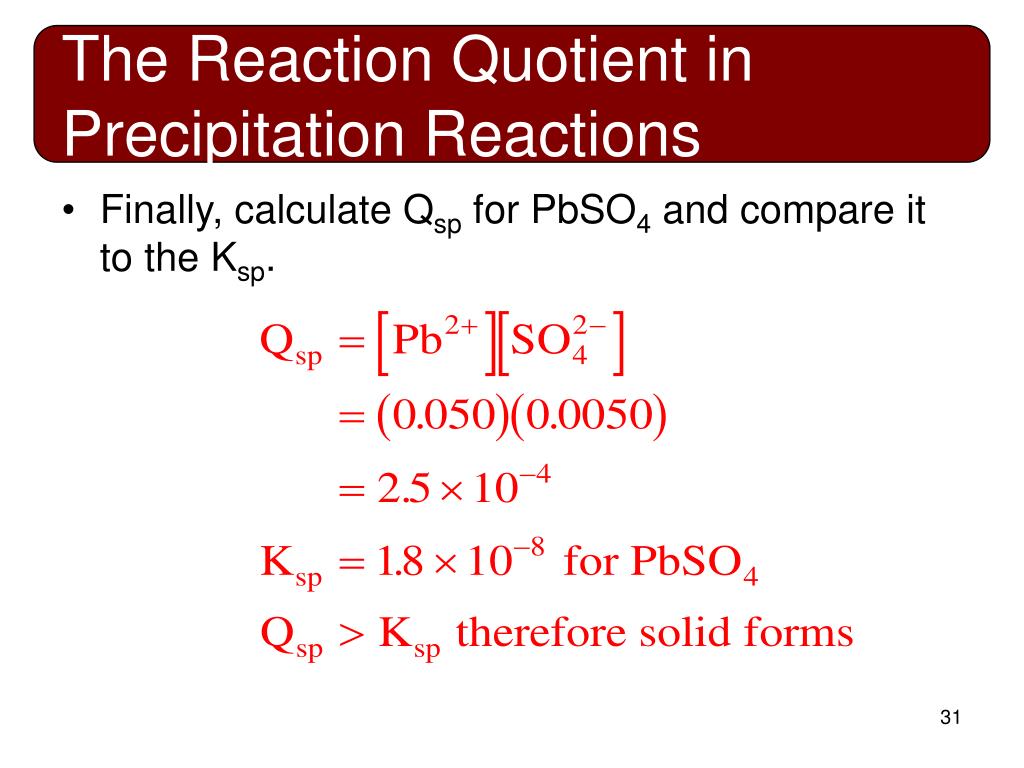
Finally, plugging into Ksp and solving, we find: x^2 = 3.36 x 10^(-9) ⇒ x = 3.3*10^(-14).. Relating Qsp and Ksp. Like regular equilibrium, we can also relate Ksp to the reaction quotient to see if a precipitation reaction will produce more or less precipitate to adjust to equilibrium. Like before, if Q > K, the reaction will produce more reactant.

Ksp 3 (Qsp vs Ksp) YouTube
The equilibrium constant for a dissolution reaction, called the solubility product ( Ksp ), is a measure of the solubility of a compound. Whereas solubility is usually expressed in terms of mass of solute per 100 mL of solvent, Ksp is defined in terms of the molar concentrations of the component ions. In contrast, the ion product ( Q) describes.